Allyl propyl disulfide
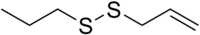 | |
| Names | |
|---|---|
| Preferred IUPAC name 3-(Propyldisulfanyl)prop-1-ene | |
| Other names | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider |
|
| ECHA InfoCard | 100.016.864 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII |
|
| UN number | 1993 |
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C6H12S2 |
| Appearance | Pale-yellow liquid |
| Odor | strong onion-like odor[1] |
| Density | 0.984 g/cm3 |
| Melting point | −15 °C; 5 °F; 258 K |
Solubility in water | Insoluble[1] |
| Hazards | |
| GHS labelling: | |
Pictograms |  |
| Warning | |
Hazard statements | H315, H319, H335 |
Precautionary statements | P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 |
| Flash point | 54.4 °C (129.9 °F; 327.5 K) |
| NIOSH (US health exposure limits): | |
PEL (Permissible) | TWA 2 ppm (12 mg/m3)[1] |
REL (Recommended) | TWA 2 ppm (12 mg/m3) ST 3 ppm (18 mg/m3)[1] |
IDLH (Immediate danger) | N.D. [1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).  N verify (what is N verify (what is  Y Y N ?) N ?) Infobox references | |
Chemical compound
Allyl propyl disulfide is an organosulfur compound with the chemical formula C3H5S2C3H7. It is a volatile pale-yellow liquid with a strong odor. It is a major component of onion oil and is used in food additives and flavors.[2]
Allyl propyl disulfide is present in garlic and onion. When onion or garlic is sliced, the substance evaporates and causes eyes to irritate.[3] When garlic or onion is cooked, it also evaporates, ridding them of the spicy taste, and leaving a sweet taste.[citation needed]
References
- ^ a b c d e f g h i j NIOSH Pocket Guide to Chemical Hazards. "#0020". National Institute for Occupational Safety and Health (NIOSH).
- ^ Lawson, Larry D.; Wang, Zhen Yu J.; Hughes, Bronwyn G. "Identification and HPLC quantitation of the sulfides and dialk(en)yl thiosulfinates in commercial garlic products" Planta Medica 1991, vol. 57, pp. 363-70. doi:10.1055/s-2006-960119
- ^ CDC - NIOSH Pocket Guide to Chemical Hazards
- v
- t
- e









