Neohesperidose
 | |
 | |
| Names | |
|---|---|
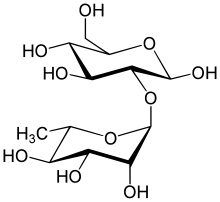
| IUPAC name α-L-Rhamnopyranosyl-(1→2)-D-glucose | |
| Systematic IUPAC name (2R,3S,4R,5R)-3,4,5,6-Tetrahydroxy-2-{[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}hexanal | |
| Other names 2-O-alpha-L-Rhamnopyranosyl-D-glucopyranose 2-O-alpha-L-Rhamnosyl-D-glucose 2-O-(6-deoxy-alpha-L-mannopyranosyl)-beta-D-glucopyranose | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| ECHA InfoCard | 100.037.379 |
| KEGG |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C12H22O10 |
| Molar mass | 326.29 g/mol |
| Density | 1.662 g/mL |
| Related compounds | |
Related compounds | Rhamnose Glucose |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).  N verify (what is N verify (what is  Y Y N ?) N ?) Infobox references | |
Chemical compound
Neohesperidose is the disaccharide which is present in some flavonoids. It can be found in species of Typha.[1] [2]
Neohesperidosides
- Cyanidin-3-neohesperidoside[2]
- Delphinidin-3-neohesperidoside[2]
- Rhoifolin or apigenin 7-O-neohesperidoside
- Myricetin-3-O-neohesperidoside found in Physalis angulata[3]
- Neohesperidin (hesperetin 7-O-neohesperidoside)
- Neoeriocitrin (eriodictyol 7-O-neohesperidoside)
See also
References
- Synthesis of neohesperidose, B. H. Koeppen, 1968[dead link]
- ^ Flavonoids of citrus—VI *1: The structure of neohesperidose, R. M. Horowitz and Bruno Gentili, 1962[dead link]
- ^ a b c Delphinidin-3-neohesperidoside and cyanidin-3- neohesperidoside from receptacles of Podocarpus species, Oyvind M. Andersen, Phytochemistry, 1989, Volume 28, Issue 2, Pages 495–497, doi:10.1016/0031-9422(89)80039-1
- ^ A novel cytotoxic flavonoid glycoside from Physalis angulata. N. Ismail and M. Alam, Fitoterapia, Volume 72, Issue 6, August 2001, Pages 676-679, doi:10.1016/S0367-326X(01)00281-7
External links
- Neohesperidose on rdchemicals.com
- v
- t
- e












